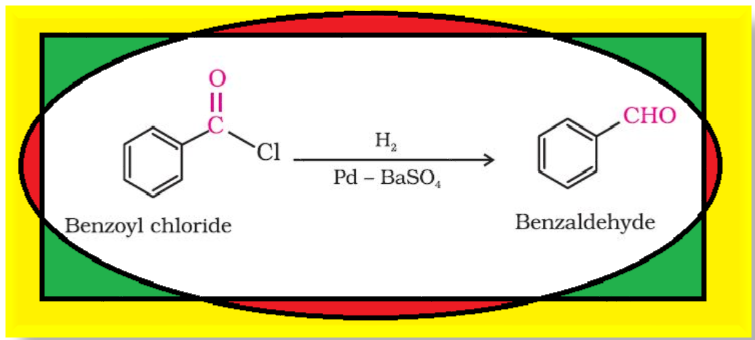
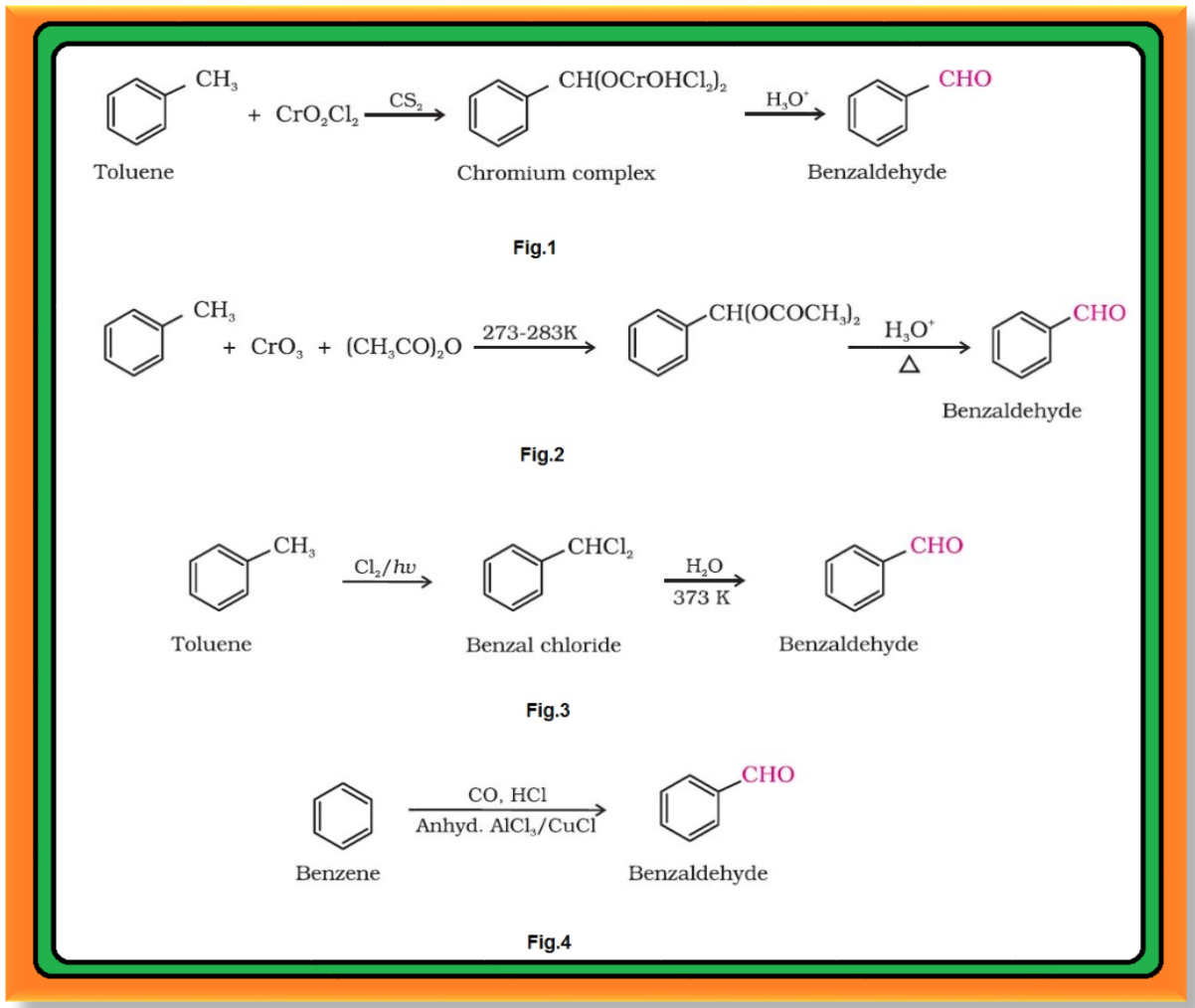
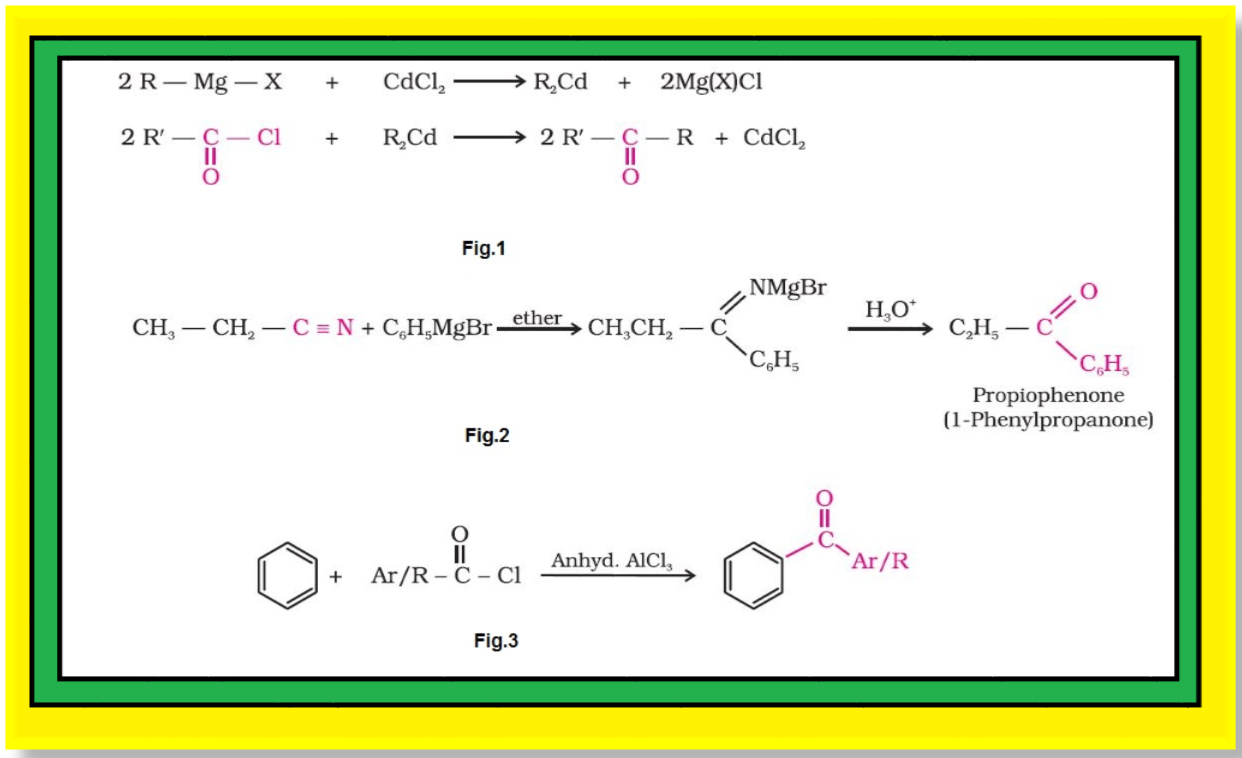
Preparation of Aldehydes and Ketones :
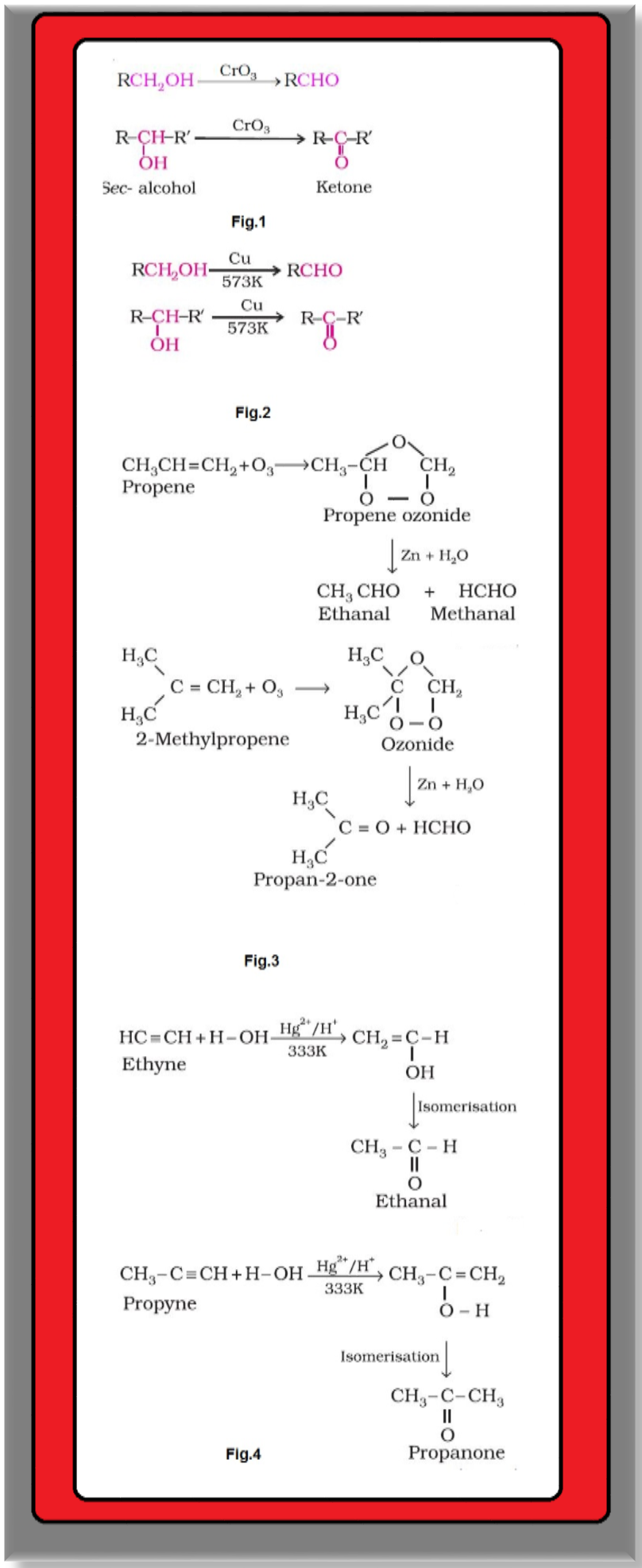
`color{green}(text(By Oxidation of Alcohols ))` : Aldehydes and ketones are generally prepared by oxidation of primary and secondary alcohols, respectively. See fig.1.
`color{green}(text(By Dehydrogenation of Alcohols ))` : This method is suitable for volatile alcohols and is of industrial application.
● In this method alcohol vapours are passed over heavy metal catalysts (`color{red}(Ag)` or `color{red}(Cu)`).
● Primary and secondary alcohols give aldehydes and ketones, respectively. See fig.2.
`color{green}(text(From Hydrocarbons ))` :
(i) `color{green}(text(By Ozonolysis of Alkenes )) ` : As we know, ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes, ketones or a mixture of both depending on the substitution pattern of the alkene. See fig.3.
(ii) `color{green}(text(By Hydration of Alkynes ))` : Addition of water to ethyne in the presence of `color{red}(H_2SO_4)` and `color{red}(HgSO_4)` gives acetaldehyde. All other alkynes give ketones in this reaction. See fig.4.
`color{green}(text(By Dehydrogenation of Alcohols ))` : This method is suitable for volatile alcohols and is of industrial application.
● In this method alcohol vapours are passed over heavy metal catalysts (`color{red}(Ag)` or `color{red}(Cu)`).
● Primary and secondary alcohols give aldehydes and ketones, respectively. See fig.2.
`color{green}(text(From Hydrocarbons ))` :
(i) `color{green}(text(By Ozonolysis of Alkenes )) ` : As we know, ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes, ketones or a mixture of both depending on the substitution pattern of the alkene. See fig.3.
(ii) `color{green}(text(By Hydration of Alkynes ))` : Addition of water to ethyne in the presence of `color{red}(H_2SO_4)` and `color{red}(HgSO_4)` gives acetaldehyde. All other alkynes give ketones in this reaction. See fig.4.